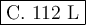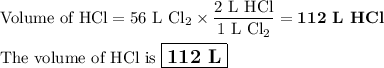Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produce...

Chemistry, 11.12.2019 02:31 yashirachevalier
Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produced when 56 l of chlorine are reacted with excess
hydrogen?
(one mole of any gas occupies 22.4 l under certain conditions of temperature and
pressure. assume those conditions for this question.)
a. 22.4l
b. 56 l
c. 112
d. 224 l

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Questions

Physics, 04.02.2021 04:30

Mathematics, 04.02.2021 04:30

Mathematics, 04.02.2021 04:30

English, 04.02.2021 04:30

Biology, 04.02.2021 04:30

Mathematics, 04.02.2021 04:30



Mathematics, 04.02.2021 04:30

Social Studies, 04.02.2021 04:30

History, 04.02.2021 04:30







Mathematics, 04.02.2021 04:30

English, 04.02.2021 04:30