
Chemistry, 11.12.2019 02:31 samueltaye
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.
co(g) + h2o(g) < => co2(g) + h2(g)(volume is decreased)pcl3(g) + cl2(g) < => pcl5(g)(volume is increased)caco3(s)< => cao(s) + co2(g)(volume is increased)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as...
Questions


Geography, 18.03.2021 02:40





Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Spanish, 18.03.2021 02:40


Computers and Technology, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Biology, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40

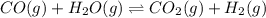 (volume is decreased)
(volume is decreased)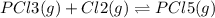 (volume is increased)
(volume is increased)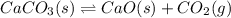 (volume is increased)
(volume is increased)

