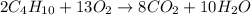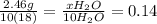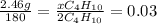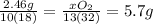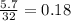The combustion of a sample of butane, c4h10, produced 2.46 grams of water.
2c4h10 + 13o2 -> 8co2 + 10h20
(a) how many moles of water formed? (b) how many moles of butane burned?
(c) how many grams of butane burned?
(d) how much oxygen was used up in moles? and in grams?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1


Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 13:00
Me puede ayudar con estas preguntas 1. diga que estudia la química orgánica explica el nacimiento bioquímicas 2. cual es la importancia de la química orgánica. 3. determine las principales características del hidrógeno, oxigeno, nitrógeno y azufre como elementos que constituyen los compuestos orgánicas. 4. elabore una tabla comparativa entre compuestas orgánicas e incaicos.
Answers: 1
You know the right answer?
The combustion of a sample of butane, c4h10, produced 2.46 grams of water.
2c4h10 + 13o2 ->...
2c4h10 + 13o2 ->...
Questions




Mathematics, 12.02.2021 20:40


Computers and Technology, 12.02.2021 20:40

Mathematics, 12.02.2021 20:40


Mathematics, 12.02.2021 20:40

Physics, 12.02.2021 20:40


Mathematics, 12.02.2021 20:40



History, 12.02.2021 20:40

Mathematics, 12.02.2021 20:40

Mathematics, 12.02.2021 20:40

Mathematics, 12.02.2021 20:40