Chemistry, 11.12.2019 04:31 4Myboyz1234
The dipole moment (μ) of hbr (a polar covalent molecule) is 0.851d (debye), and its percent ionic character is 12.6 % . estimate the bond length of the h−br bond in picometers. note that 1 d=3.34×10−30 c⋅m and

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
The dipole moment (μ) of hbr (a polar covalent molecule) is 0.851d (debye), and its percent ionic ch...
Questions


Mathematics, 04.11.2020 19:00



Social Studies, 04.11.2020 19:00





Arts, 04.11.2020 19:00

Mathematics, 04.11.2020 19:00



Mathematics, 04.11.2020 19:00





Geography, 04.11.2020 19:00

Mathematics, 04.11.2020 19:00

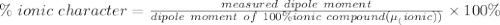
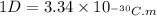
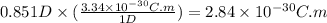
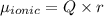
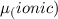 as follows:
as follows: