
Chemistry, 11.12.2019 04:31 maguilarz2005
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specific heats of ice, water, and steam are 2.03 j/g · °c, 4.184 j/g · °c, and 1.99 j/g · °c, respectively. the heat of fusion of water is 6.01 kj/mol, the heat of vaporization is 40.79 kj/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
How much heat (in kj) is needed to convert 856 g of ice at −10.0°c to steam at 126.0°c? (the specif...
Questions

Social Studies, 27.12.2021 19:40


English, 27.12.2021 19:40



Social Studies, 27.12.2021 19:40


Computers and Technology, 27.12.2021 19:40


History, 27.12.2021 19:40







SAT, 27.12.2021 19:40

Social Studies, 27.12.2021 19:40


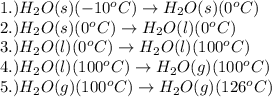
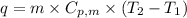 .......(1)
.......(1)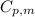 = specific heat capacity of medium
= specific heat capacity of medium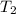 = final temperature
= final temperature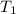 = initial temperature
= initial temperature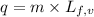 ......(2)
......(2)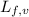 = latent heat of fusion or vaporization
= latent heat of fusion or vaporization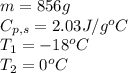
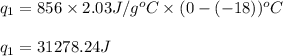
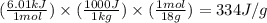
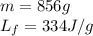
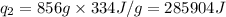
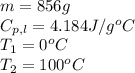
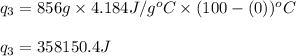
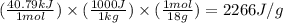
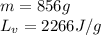
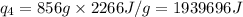
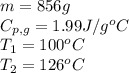
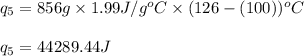
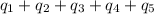
![[31278.24+285904+358150.4+1939696+44289.44]J=2659318.08J=2659.3kJ](/tpl/images/0412/9728/2c085.png)


