
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volume of 3.4 l. a. if the container is evacuated (all of the gas removed), sealed, and then allowed to warm to room temperature t = 298 k so that all of the solid co2 is converted to a gas, what is the pressure inside the container?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
A41.1 g sample of solid co2 (dry ice) is added to a container at a temperature of 100 k with a volum...
Questions

Biology, 06.05.2020 07:01


Mathematics, 06.05.2020 07:01

Social Studies, 06.05.2020 07:01




History, 06.05.2020 07:01

Chemistry, 06.05.2020 07:01

SAT, 06.05.2020 07:01


Computers and Technology, 06.05.2020 07:01


Mathematics, 06.05.2020 07:01



Mathematics, 06.05.2020 07:01



Business, 06.05.2020 07:01

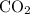 :
: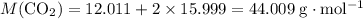 .
.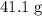 sample of
sample of 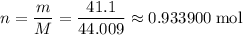 .
.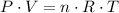 ,
, 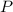 is the pressure inside the container.
is the pressure inside the container.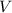 is the volume of the container.
is the volume of the container.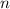 is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.
is the number of moles of particles (molecules, or atoms in case of noble gases) in the gas.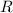 is the ideal gas constant.
is the ideal gas constant. 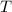 is the absolute temperature of the gas.
is the absolute temperature of the gas.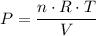 .
.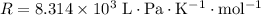 .
.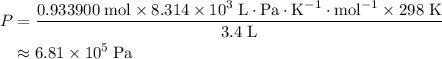 .
.

