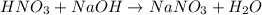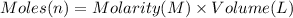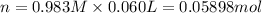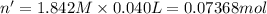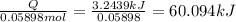Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3


Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
In a calorimeter, you combine 60 ml of a 0.983 m hno3 solution with 40 ml of a 1.842 m naoh solution...
Questions



Social Studies, 18.07.2019 20:50

Social Studies, 18.07.2019 20:50



Arts, 18.07.2019 20:50

Biology, 18.07.2019 20:50





Social Studies, 18.07.2019 20:50

Computers and Technology, 18.07.2019 20:50

Spanish, 18.07.2019 20:50

History, 18.07.2019 20:50



Geography, 18.07.2019 20:50