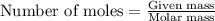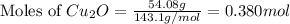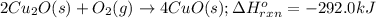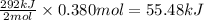Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions


Mathematics, 11.10.2019 09:10

Social Studies, 11.10.2019 09:10



Mathematics, 11.10.2019 09:10

Mathematics, 11.10.2019 09:10

Mathematics, 11.10.2019 09:10





Geography, 11.10.2019 09:10



Mathematics, 11.10.2019 09:10

Mathematics, 11.10.2019 09:10


Computers and Technology, 11.10.2019 09:10

Mathematics, 11.10.2019 09:10

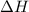 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ