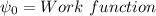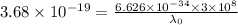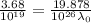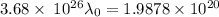Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Aforce of attraction/repulsion due to the spin of electrons what is this force?
Answers: 2

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
For potassium metal, the work function φ (the minimum energy needed to eject an electron from the me...
Questions



Mathematics, 20.04.2021 19:30

Biology, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30


Mathematics, 20.04.2021 19:30

History, 20.04.2021 19:30


Biology, 20.04.2021 19:30

Mathematics, 20.04.2021 19:30




Mathematics, 20.04.2021 19:30



Mathematics, 20.04.2021 19:30


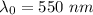
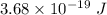
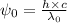
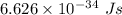
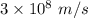
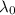 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded