
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its natural abundance is only 0.72 percent. to separate it from the more abundant 238u isotope (atomic mass = 238.00), uranium is first converted to uf6, which is easily vaporized above room temperature. the mixture of 235uf6 and 238uf6 gases is then subjected to many stages of effusion. calculate how much more quickly 235uf6 effuses than 238uf6. give the answer as the ratio of rates of 235uf6 to 238uf6 to four decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
The 235u isotope (atomic mass = 235.00) undergoes fission when bombarded with neutrons. however, its...
Questions

Mathematics, 15.02.2021 22:40



Biology, 15.02.2021 22:40

Mathematics, 15.02.2021 22:40

English, 15.02.2021 22:40

English, 15.02.2021 22:40



Mathematics, 15.02.2021 22:40


Biology, 15.02.2021 22:40



English, 15.02.2021 22:40



Geography, 15.02.2021 22:40

History, 15.02.2021 22:40

English, 15.02.2021 22:40

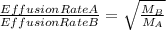
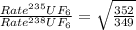 = 1.0043
= 1.0043

