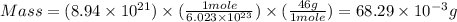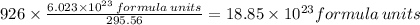Chemistry, 11.12.2019 06:31 myleefaustin
What are the steps to solving these problems? i don’t need answers, just the steps on how to get the answers.
1. find the molecular mass of calcium citrate.
4. what is the mass of 8.94 x 10^21 molecules of ethanol (c2h5oh)?
5. how many formula units are in 926 g of febr3?
!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1


Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
What are the steps to solving these problems? i don’t need answers, just the steps on how to get th...
Questions

Geography, 30.11.2020 18:50

Social Studies, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50


Mathematics, 30.11.2020 18:50


Medicine, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50

English, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50



Mathematics, 30.11.2020 18:50




Social Studies, 30.11.2020 18:50


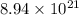 molecules of ethanol is
molecules of ethanol is 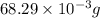
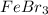 has
has 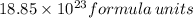
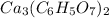
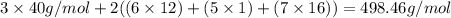
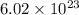 molecules.
molecules.