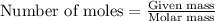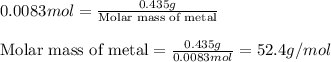A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4 ( aq ) ⟶ mso 4 ( aq ) + h 2 ( g ) a volume of 201 ml of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. atmospheric pressure is 756.0 torr, and the temperature is 25 °c. calculate the molar mass of the metal.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
A0.435 g sample of a metal, m, reacts completely with sulfuric acid according to m ( s ) + h 2 so 4...
Questions


Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50



English, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

Arts, 20.01.2021 20:50


Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50



Mathematics, 20.01.2021 20:50


Social Studies, 20.01.2021 20:50

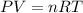
![25^oC=[25+273]K=298K](/tpl/images/0413/1404/df1f6.png)



 of metal
of metal