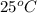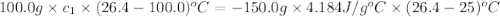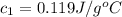Chemistry, 11.12.2019 18:31 lilquongohard
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter containing 150. g of water at 25.0 oc. after the metal cools, the final temperature of the metal and the water is 26.4 oc. calculate the specific heat capacity of lead from these experimental data, assuming that no heat escapes to the surroundings or is transferred to the calorimeter. specific heat of water = 4.184 j/g oc atomic mass pb = 207.2 g/mol calculate your answer in j/g oc with 3 significant figures, but enter the answer without units.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

You know the right answer?
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter con...
Questions

Arts, 11.06.2021 05:20

English, 11.06.2021 05:20



Mathematics, 11.06.2021 05:20



Mathematics, 11.06.2021 05:20



Mathematics, 11.06.2021 05:20

Mathematics, 11.06.2021 05:20

Geography, 11.06.2021 05:20



Mathematics, 11.06.2021 05:20

Chemistry, 11.06.2021 05:20


English, 11.06.2021 05:20

Mathematics, 11.06.2021 05:20

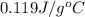
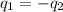

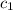 = specific heat of lead = ?
= specific heat of lead = ?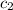 = specific heat of water =
= specific heat of water = 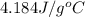
 = mass of lead = 100.0 g
= mass of lead = 100.0 g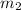 = mass of water = 150.0 g
= mass of water = 150.0 g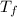 = final temperature =
= final temperature = 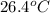
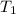 = initial temperature of lead =
= initial temperature of lead = 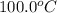
 = initial temperature of water =
= initial temperature of water =