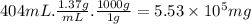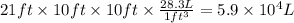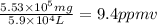Chemistry, 11.12.2019 18:31 serenityarts123
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measuring 21 ft x 10 ft x 10 ft. assuming that the contents of the beaker completely evaporate and fill the space, what is the resulting concentration in parts per million by volume (ppmv)? assume normal temperature and pressure (ntp), i. e., p = 1 atm, and t = 25 celsius. 1 ft3 = 28.3 l

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measu...
Questions

Biology, 13.02.2021 16:20


Mathematics, 13.02.2021 16:20

Geography, 13.02.2021 16:20

Mathematics, 13.02.2021 16:20


Health, 13.02.2021 16:20

Biology, 13.02.2021 16:20

Mathematics, 13.02.2021 16:30



Social Studies, 13.02.2021 16:30

Mathematics, 13.02.2021 16:30





Mathematics, 13.02.2021 16:30

Computers and Technology, 13.02.2021 16:30

English, 13.02.2021 16:30