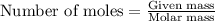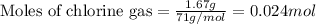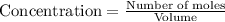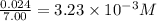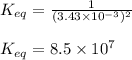Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at a certain temperature, a chemist finds that a 7.0 l reaction vessel containing a mixture of titanium, chlorine, and titanium(iv) chloride at equilibrium has the following composition compound amount 1.67 g cl 2.93 g tici 2.02 g ti calculate the value of the equilibrium constant k for this reaction. round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at...
Questions


Mathematics, 08.07.2019 13:00

Mathematics, 08.07.2019 13:00

Mathematics, 08.07.2019 13:00



Mathematics, 08.07.2019 13:00


Mathematics, 08.07.2019 13:00

Mathematics, 08.07.2019 13:00



History, 08.07.2019 13:00



Geography, 08.07.2019 13:00

Social Studies, 08.07.2019 13:00

History, 08.07.2019 13:00

Social Studies, 08.07.2019 13:00


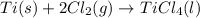
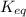 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{1}{[Cl_2]^2}](/tpl/images/0413/8706/8ac7b.png)