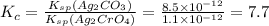Chemistry, 11.12.2019 19:31 mariahrpoulin1511
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 × 10‒12. from this data, what is the value of kc for the reaction, ag2co3(s) + cro42‒(aq) → ag2cro4(s) + co32‒(aq) a) 9.6 × 10‒12 b) 7.7 c) 1.1 × 1023 d) 1.3 × 10‒1 e) 9.4 × 10‒24

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
When molecules of water vapor in the air come in contact with a cold can of soft drink , they lose energy ,slow down,and form a liquid due to decrease in ?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
The value of the solubility product constant for ag2co3 is 8.5 × 10‒12 and that of ag2cro4 is 1.1 ×...
Questions

Chemistry, 11.07.2019 02:00

Social Studies, 11.07.2019 02:00


English, 11.07.2019 02:00

History, 11.07.2019 02:00





Biology, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Biology, 11.07.2019 02:00

Social Studies, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00



Geography, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

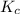 for the given reaction is 7.7
for the given reaction is 7.7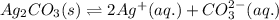
![K_{sp}(Ag_{2}CO_{3})=[Ag^{+}]^{2}[CO_{3}^{2-}]](/tpl/images/0413/8617/b8832.png)
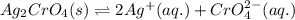
![K_{sp}(Ag_{2}CrO_{4})=[Ag^{+}]^{2}[CrO_{4}^{2-}]](/tpl/images/0413/8617/31f53.png)
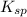 represents solubility product
represents solubility product![K_{c}=\frac{[CO_{3}^{2-}]}{[CrO_{4}^{2-}]}](/tpl/images/0413/8617/fea9e.png) (concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)
(concentration of pure solids remain constant during reaction. Hence their concentration is taken as 1 to exclude them from equilibrium constant expression)![K_{c}=\frac{[Ag^{+}]^{2}[CO_{3}^{2-}]}{[Ag^{+}]^{2}[CrO_{4}^{2-}]}](/tpl/images/0413/8617/3fc1b.png)