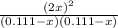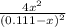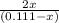Chemistry, 11.12.2019 19:31 ImBADatmath8743
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the reaction is: br2(g) + f2(g) ⇔ 2 brf(g) what is the equilibrium concentration of fluorine if the initial concentrations of bromine and fluorine were 0.111 moles/liter in a sealed container and no product was present initially?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

You know the right answer?
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the...
Questions




Social Studies, 02.08.2019 20:30


Mathematics, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30

English, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30



Mathematics, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30


Social Studies, 02.08.2019 20:30


Business, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30

![\frac{[BrF ]^{2} }{[ F_{2} ][Br_{2} ]}](/tpl/images/0413/8603/382cd.png)