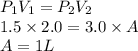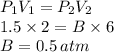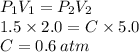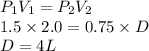Chemistry, 11.12.2019 19:31 preservations
How well can you apply boyle’s law to this sample of gas that experiences changes in pressure and volume? assume that temperature and number of moles of gas are constant in this problem.
using the first volume and pressure reading on the table as v1 and p1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1


Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
How well can you apply boyle’s law to this sample of gas that experiences changes in pressure and vo...
Questions

Mathematics, 06.02.2021 05:10

Computers and Technology, 06.02.2021 05:10








Mathematics, 06.02.2021 05:10


Arts, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10




Mathematics, 06.02.2021 05:10

English, 06.02.2021 05:10