
Chemistry, 11.12.2019 20:31 brainist71
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of iodine. i2(g) equilibrium reaction arrow 2 i(g); kc = 1.35 ✕ 10−3 suppose this reaction is initiated in a 3.7 l container with 0.063 mol i2 at 970 k. calculate the concentrations of i2 and i at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of i...
Questions




Mathematics, 28.05.2021 01:00




Computers and Technology, 28.05.2021 01:00


Mathematics, 28.05.2021 01:00


English, 28.05.2021 01:00



Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Computers and Technology, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00


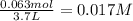
![Kc=1.35 \times 10^{-3} =\frac{[I]^{2} }{[I_{2}]} =\frac{(2x)^{2} }{(0.017-x)} \\4x^{2} +1.35 \times 10^{-3}x - 2.3 \times 10^{-5}](/tpl/images/0413/9700/35688.png)


