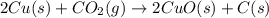The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is true?
a) the reaction is not spontaneous at any temperature.
b) the reaction is spontaneous at all temperatures.
c) it is impossible to determine if the reaction is spontaneous without calculations.
d) the reaction will be spontaneous only at low temperatures.
e) the reaction will be spontaneous only at high temperatures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

You know the right answer?
The reaction 2cu(s) + co2(g) → 2cuo(s) + c(s) is endothermic. which of the following statements is t...
Questions



Physics, 19.10.2019 01:40


History, 19.10.2019 01:40

Mathematics, 19.10.2019 01:40

Mathematics, 19.10.2019 01:40

English, 19.10.2019 01:40



History, 19.10.2019 01:40

Social Studies, 19.10.2019 01:40








Mathematics, 19.10.2019 01:40