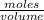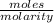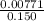Chemistry, 11.12.2019 22:31 ginger87771
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2 co 3 solution according to this balanced chemical equation?
na 2 co 3 ( a q ) + 2 hno 3 ( a q ) → 2 nano 3 ( a q ) + co 2 ( g ) + h 2 o ( l )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2...
Questions



Computers and Technology, 03.11.2020 19:10

History, 03.11.2020 19:10


Health, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10




Mathematics, 03.11.2020 19:10




Computers and Technology, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10



Mathematics, 03.11.2020 19:10