Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
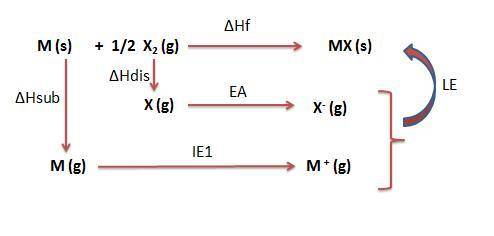
Calculate the lattice energy (kj/mol) of the hypothetical ionic compound mx from the information giv...
Questions


Mathematics, 07.09.2021 21:50


Mathematics, 07.09.2021 21:50

Mathematics, 07.09.2021 21:50

Mathematics, 07.09.2021 21:50



Business, 07.09.2021 21:50

Mathematics, 07.09.2021 21:50

English, 07.09.2021 21:50



Mathematics, 07.09.2021 21:50






Mathematics, 07.09.2021 21:50