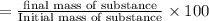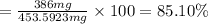Chemistry, 11.12.2019 23:31 Mypasswordishotdog11
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain = 64.7989 mg). if your tablet had a mass of 516 mg, calculate the percent recovery if you isolated 386 mg of acetylsalicylic acid in this experiment.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain...
Questions

Spanish, 09.07.2019 00:30



Mathematics, 09.07.2019 00:30

History, 09.07.2019 00:30

Mathematics, 09.07.2019 00:30


Physics, 09.07.2019 00:30




Computers and Technology, 09.07.2019 00:30

Mathematics, 09.07.2019 00:30


History, 09.07.2019 00:30

Mathematics, 09.07.2019 00:30

Mathematics, 09.07.2019 00:30


Mathematics, 09.07.2019 00:30