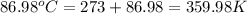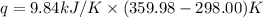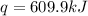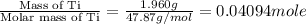Chemistry, 11.12.2019 23:31 cheyennemitchel2680
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 86.98 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2


Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
When 1.960 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter incr...
Questions


Mathematics, 22.07.2019 09:00



Mathematics, 22.07.2019 09:00










Mathematics, 22.07.2019 09:00





Mathematics, 22.07.2019 09:00

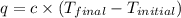
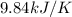
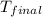 = final temperature =
= final temperature = 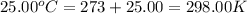
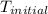 = initial temperature =
= initial temperature =