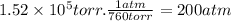Chemistry, 12.12.2019 00:31 ZoomZoom44
There are limitations to the amount of pressure that can be applied during reverse osmosis because of the physical nature of the membrane. a membrane has been developed that can withstand osmotic pressures, π, of up to 1.52×105 torr . calculate the maximum molar concentration of a solution that can be purified by reverse osmosis using this membrane at 25 ∘c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
There are limitations to the amount of pressure that can be applied during reverse osmosis because o...
Questions


Mathematics, 06.11.2020 03:20



Biology, 06.11.2020 03:20

Mathematics, 06.11.2020 03:20



Mathematics, 06.11.2020 03:20

Chemistry, 06.11.2020 03:20

History, 06.11.2020 03:20


Mathematics, 06.11.2020 03:20

History, 06.11.2020 03:20

Mathematics, 06.11.2020 03:20


Mathematics, 06.11.2020 03:20

Social Studies, 06.11.2020 03:20

Mathematics, 06.11.2020 03:20