
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl 2 ( g ) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl 3 ( g ) + cl 2 ( g ) − ⇀ ↽ − pcl 5 ( g ) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
An equilibrium mixture of pcl 5 ( g ) , pcl 3 ( g ) , and cl 2 ( g ) has partial pressures of 217.0...
Questions



Mathematics, 19.09.2019 22:30


Social Studies, 19.09.2019 22:30





Arts, 19.09.2019 22:30


Health, 19.09.2019 22:30


Physics, 19.09.2019 22:30




Biology, 19.09.2019 22:30

Social Studies, 19.09.2019 22:30

 when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.
when equilibrium is re-established are 223.4 torr, 6.82 torr and 26.4 torr respectively.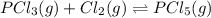
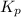 for above reaction follows:
for above reaction follows: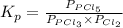 ........(1)
........(1)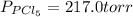
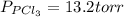
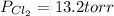
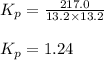
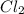 .
.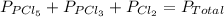
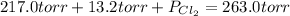
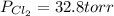
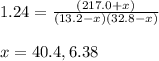
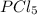 = (217.0+x) = (217.0+6.38) = 223.4 torr
= (217.0+x) = (217.0+6.38) = 223.4 torr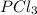 = (13.2-x) = (13.2-6.38) = 6.82 torr
= (13.2-x) = (13.2-6.38) = 6.82 torr


