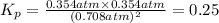Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Areaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen a...
Questions



Mathematics, 12.12.2019 04:31



SAT, 12.12.2019 04:31

English, 12.12.2019 04:31


English, 12.12.2019 04:31


Mathematics, 12.12.2019 04:31



Mathematics, 12.12.2019 04:31



Mathematics, 12.12.2019 04:31

Chemistry, 12.12.2019 04:31