Chemistry, 12.12.2019 03:31 ayoismeisjjjjuan
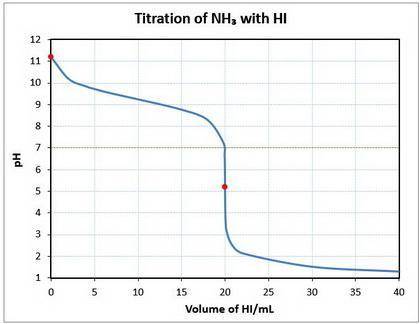
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0 ml of a solution of 0.150 mol/l of the strong acid hydroiodic acid (hi (
a) write a balanced equation for the titration reaction.
b) what is the ph of the ammonia solution before the titration begins?
c) what is the ph at the equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
10. a 20.00 ml sample of 0.150 mol/l ammonia (nh3(aq)) is titrated to the equivalence point by 20.0...
Questions





History, 31.07.2019 23:30

Business, 31.07.2019 23:30

Mathematics, 31.07.2019 23:30


Mathematics, 31.07.2019 23:30

Mathematics, 31.07.2019 23:30



Advanced Placement (AP), 31.07.2019 23:30




Chemistry, 31.07.2019 23:30

Mathematics, 31.07.2019 23:30


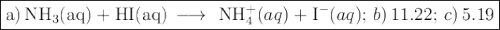

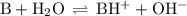
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.150 - x} = 1.8 \times 10^{-5}](/tpl/images/0414/6924/2256c.png)
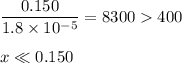
![\dfrac{x^{2}}{0.150} = 1.8 \times 10^{-5}\\\\x^{2} = 0.150 \times 1.8 \times 10^{-5}\\x^{2} = 2.7 \times 10^{-6}\\x = \sqrt{2.7 \times 10^{-6}}\\x = \text{[OH]}^{-} = 1.64 \times 10^{-3} \text{ mol/L}](/tpl/images/0414/6924/0b94c.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.64 \times 10^{-3}) = 2.78\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.78 = \mathbf{11.22}\\\\\text{The pH of the solution at equilibrium is } \large \boxed{\mathbf{11.22}}](/tpl/images/0414/6924/f4986.png)
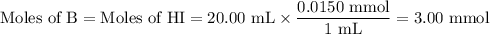
![\rm [BH^{+}] = \dfrac{\text{3.00 mmol}}{\text{40.00 mL}} = \text{0.0750 mol/L}](/tpl/images/0414/6924/607b2.png)
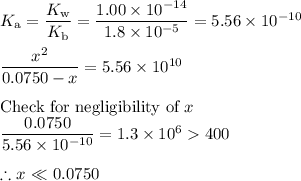
![\dfrac{x^{2}}{0.0750} = 5.56 \times 10^{-10}\\\\x^{2} = 0.0750 \times 5.56 \times 10^{-10}\\x^{2} = 4.17 \times 10^{-11}\\x = \sqrt{4.17 \times 10^{-11}}\\\rm [H_{3}O^{+}] =x = 6.46 \times 10^{-6}\, mol \cdot L^{-1}](/tpl/images/0414/6924/dc4d9.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{6.46 \times 10^{-6}} = \large \boxed{\mathbf{5.19}}](/tpl/images/0414/6924/b2d28.png)