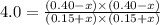Chemistry, 12.12.2019 03:31 antoniaannswiney
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the equilibrium constant for this reaction with dioxane as a solvent is 4.0. what are the equilibrium concentrations for a mixture that is initially 0.15 m in ch3co2h, 0.15 m in c2h5oh, 0.40 m in ch3co2c2h5, and 0.40 m in h2o?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

You know the right answer?
Acetic acid, ch3co2h, reacts with ethanol, c2h5oh, to form water and ethyl acetate, ch3co2c2h5. the...
Questions


Computers and Technology, 11.12.2021 06:20

Mathematics, 11.12.2021 06:20

History, 11.12.2021 06:20



Mathematics, 11.12.2021 06:20

Mathematics, 11.12.2021 06:20

English, 11.12.2021 06:20

Mathematics, 11.12.2021 06:20



Geography, 11.12.2021 06:20


Mathematics, 11.12.2021 06:20

History, 11.12.2021 06:20

Computers and Technology, 11.12.2021 06:20

Mathematics, 11.12.2021 06:20


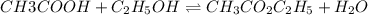
![K_c=\frac{[CH_3CO_2C_2H_5][H_2O]}{[CH_3COOH][C_2H_5OH]}](/tpl/images/0414/6294/2612b.png)