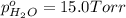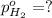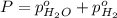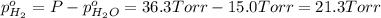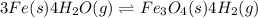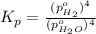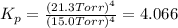Chemistry, 12.12.2019 04:31 makalaily9342
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pressure of water vapor is 15.0 torr, the total pressure at equilibrium is 36.3 torr. 3 fe(s) 4 h2o(g) equilibrium reaction arrow fe3o4(s) 4 h2(g) calculate the value of kp for this reaction at 1200 k. hint: apply dalton's law of partial pressures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
In a study of the following reaction at 1200 k it was observed that when the equilibrium partial pre...
Questions




Physics, 24.12.2019 03:31




Chemistry, 24.12.2019 03:31

Social Studies, 24.12.2019 03:31



Chemistry, 24.12.2019 03:31

Law, 24.12.2019 03:31

Social Studies, 24.12.2019 03:31




Medicine, 24.12.2019 03:31


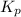 for this reaction at 1200 K is 4.066.
for this reaction at 1200 K is 4.066.