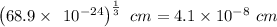The density of solid ag is 10.5 g/cm3. how many atoms are present per cubic centimeter of ag?
as a solid, ag adopts a face-centered cubic unit cell. how many unit cells are present per cubic centimeter of ag?
what is the volume of a unit cell of this metal?
what is the edge length of a unit cell of ag?
break it down to me step-by-step, so i can understand it.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3


Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The density of solid ag is 10.5 g/cm3. how many atoms are present per cubic centimeter of ag?
Questions

Mathematics, 17.07.2020 05:01


English, 17.07.2020 05:01





Medicine, 17.07.2020 05:01




History, 17.07.2020 05:01

Mathematics, 17.07.2020 05:01





Mathematics, 17.07.2020 05:01


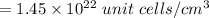
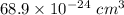
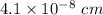
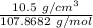 =0.0973 mol/cm³
=0.0973 mol/cm³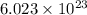 atoms.
atoms.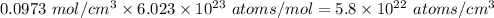
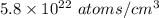
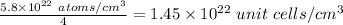
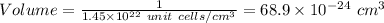
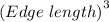
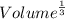 =
=