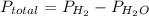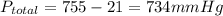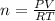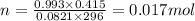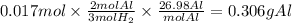Chemistry, 12.12.2019 04:31 paigebmaxwell6062
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is allowed to react, 415ml of gas is collected over water at 23°c at a pressure of 755mmhg. at 23°c the vapor pressure of water is 21mmhg. what is the pressure in mmhg of the dry h2 gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

You know the right answer?
Solid aluminum reacts with aqueous h2so4 to form h2 gas and aluminum sulfate. when a sample of al is...
Questions


Mathematics, 02.04.2021 23:20


Social Studies, 02.04.2021 23:20


Mathematics, 02.04.2021 23:20


Mathematics, 02.04.2021 23:20



Mathematics, 02.04.2021 23:20

Mathematics, 02.04.2021 23:20


History, 02.04.2021 23:20

Advanced Placement (AP), 02.04.2021 23:20

Mathematics, 02.04.2021 23:20


Social Studies, 02.04.2021 23:20


Chemistry, 02.04.2021 23:20