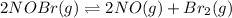Chemistry, 12.12.2019 04:31 dhurtado1195
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reaction is endothermic. what do you expect to happen to the concentration of no if the temperature is doubled? 2nobr( g) ⇌ 2no( g) + br 2( g) the change in concentration of [no] will depend on the size of the vessel. the concentration of no will increase. the concentration of no will decrease. a catalyst will be needed to make a change in concentration. there will be no change in [no].

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1



You know the right answer?
For the following reaction, the equilibrium constant kc is 2.0 at a certain temperature. the reactio...
Questions

Mathematics, 29.05.2021 14:00

English, 29.05.2021 14:00



Mathematics, 29.05.2021 14:00


Physics, 29.05.2021 14:00


Chemistry, 29.05.2021 14:00


Geography, 29.05.2021 14:00


Computers and Technology, 29.05.2021 14:00

Mathematics, 29.05.2021 14:00

Physics, 29.05.2021 14:00

Mathematics, 29.05.2021 14:00

Mathematics, 29.05.2021 14:00


Mathematics, 29.05.2021 14:00

Physics, 29.05.2021 14:00