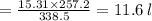Chemistry, 12.12.2019 05:31 laykaleb086
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the temperature is –15.8°c and its pressure is 524 torr? 20.2 l 11.6 l 63.5 l not possible, since the volume would have to be negative 3.69 l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Asample of ammonia gas at 65.5°c and 524 torr has a volume of 15.31 l. what is its volume when the t...
Questions


English, 28.03.2021 14:00


Computers and Technology, 28.03.2021 14:00

Mathematics, 28.03.2021 14:00

Mathematics, 28.03.2021 14:00

Physics, 28.03.2021 14:00

Chemistry, 28.03.2021 14:00

Chemistry, 28.03.2021 14:00



Mathematics, 28.03.2021 14:00



Mathematics, 28.03.2021 14:00

Chemistry, 28.03.2021 14:00

Health, 28.03.2021 14:00


Mathematics, 28.03.2021 14:00