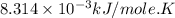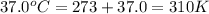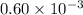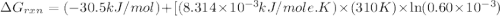Chemistry, 12.12.2019 05:31 eileentennyson
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp ( aq ) + h 2 o ( l ) ⟶ adp ( aq ) + hpo 2 − 4 ( aq ) for which δ g ∘ rxn = − 30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ g rxn in a biological cell in which [ atp ] = 5.0 mm, [ adp ] = 0.60 mm, and [ hpo 2 − 4 ] = 5.0 mm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Biology, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Social Studies, 18.11.2020 20:50

English, 18.11.2020 20:50

Biology, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Advanced Placement (AP), 18.11.2020 20:50

Chemistry, 18.11.2020 20:50



Mathematics, 18.11.2020 20:50

History, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50



Health, 18.11.2020 20:50


Health, 18.11.2020 20:50

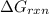 is -49.6 kJ/mol
is -49.6 kJ/mol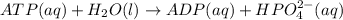
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0414/8690/ccdf0.png)
![[ATP]](/tpl/images/0414/8690/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0414/8690/68360.png) = 0.60 mM
= 0.60 mM![[HPO_4^{2-}]](/tpl/images/0414/8690/c0ca9.png) = 5.0 mM
= 5.0 mM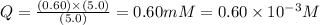
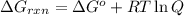 ............(1)
............(1)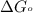 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol