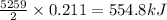Chemistry, 12.12.2019 06:31 alexdziob01
)b5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released when 0.211 mol of b5h9 reacts with excess oxygen where the products are b2o3(s) and h2o(l). the standard enthalpy of formation of b5h9(l) is 73.2 kj/mol, the standard enthalpy of formation of b2o3(s) is -1272 kj/mol and that of h2o(l) is -285.4 kj/mol. express your answer in kj.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
)b5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released w...
Questions

Mathematics, 20.08.2021 01:00


English, 20.08.2021 01:00


Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

Mathematics, 20.08.2021 01:00

Business, 20.08.2021 01:00


Mathematics, 20.08.2021 01:00


Mathematics, 20.08.2021 01:00





Mathematics, 20.08.2021 01:00


Mathematics, 20.08.2021 01:00

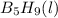 reacts is 554.8 kJ
reacts is 554.8 kJ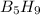 with oxygen gas follows:
with oxygen gas follows: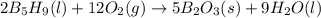
![\Delta H_{rxn}=[(5\times \Delta H_f_{(B_2O_3(s))})+(9\times \Delta H_f_{(H_2O(l))})]-[(2\times \Delta H_f_{(B_5H_9(l))})+(12\times \Delta H_f_{(O_2(g))})]](/tpl/images/0414/9485/05a9d.png)
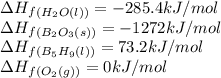
![\Delta H_{rxn}=[(2\times (-1272))+(9\times (-285.4))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H_{rxn}=-5259kJ](/tpl/images/0414/9485/eaac9.png)