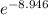Given that the free energy of the twist-boat conformer of cyclohexane is 5.3 kcal/mol greater than that of the chair conformer, calculate the percentage of twist-boat conformers present in a sample of cyclohexane at 25 °c. does your answer agree with the statement made about the relative number of molecules in these two conformations?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

You know the right answer?
Given that the free energy of the twist-boat conformer of cyclohexane is 5.3 kcal/mol greater than t...
Questions



Advanced Placement (AP), 20.11.2020 21:20

Mathematics, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20


History, 20.11.2020 21:20

English, 20.11.2020 21:20

History, 20.11.2020 21:20



History, 20.11.2020 21:20



History, 20.11.2020 21:20

Biology, 20.11.2020 21:20

History, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20