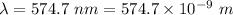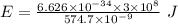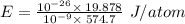Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Given that the wavelength of maximum absorption for chromium is 574.7 nm, what is the energy per cr...
Questions

Physics, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31




Mathematics, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31


History, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31

Arts, 24.01.2020 07:31


Mathematics, 24.01.2020 07:31


English, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31

Mathematics, 24.01.2020 07:31


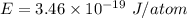
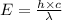
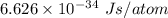
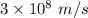
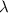 is the wavelength of the light
is the wavelength of the light