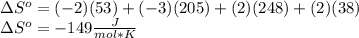Chemistry, 13.12.2019 01:31 kaylynnstanley22
Given the standard entropy values in the table, what is the value of `delta"sº"_"reaction"` for the following reaction?
2nis(s) + 3o2(g) → 2so2(g) + 2nio(s)
a. –149 j/mol·k
b. +149 j/mol·k
c. +28 j/mol·k
d. – 28 j/mol·k


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Given the standard entropy values in the table, what is the value of `delta"sº"_"reaction"` for the...
Questions


Mathematics, 02.11.2020 22:30



Mathematics, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30


English, 02.11.2020 22:30







Health, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30



Mathematics, 02.11.2020 22:30

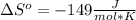
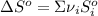
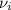 is the stoichiometric coefficient of the species i which is negative for products and positive for reactants and
is the stoichiometric coefficient of the species i which is negative for products and positive for reactants and 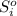 is the standard entropy for the species i in J/mol*K. Thus, we have:
is the standard entropy for the species i in J/mol*K. Thus, we have: