Consider the following chemical equation. how many moles of seo3 can be produced from 35
g of...

Chemistry, 13.12.2019 01:31 karinagramirezp072gb
Consider the following chemical equation. how many moles of seo3 can be produced from 35
g of oxygen and excess selenium?
25e +302 - 25e03

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Questions



English, 28.06.2019 19:30


Biology, 28.06.2019 19:30


Mathematics, 28.06.2019 19:30




Mathematics, 28.06.2019 19:30



History, 28.06.2019 19:30

Geography, 28.06.2019 19:30


Chemistry, 28.06.2019 19:30



History, 28.06.2019 19:30

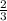 moles of SeO3
moles of SeO3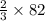 g of SeO3
g of SeO3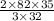 g of SeO3
g of SeO3 

