
Chemistry, 13.12.2019 02:31 Xavierayala2003
Using the following thermochemical data, what is the change in enthalpy for the following reaction?
a.
120.9 kj
b.
-251.1 kj
c.
251.1 kj
d.
-120.9 kj


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1
You know the right answer?
Using the following thermochemical data, what is the change in enthalpy for the following reaction?...
Questions

History, 08.03.2021 14:00


Mathematics, 08.03.2021 14:00







Social Studies, 08.03.2021 14:00

Social Studies, 08.03.2021 14:00

Mathematics, 08.03.2021 14:00

Social Studies, 08.03.2021 14:00

English, 08.03.2021 14:00

Physics, 08.03.2021 14:00

Physics, 08.03.2021 14:00

Physics, 08.03.2021 14:00

Health, 08.03.2021 14:00


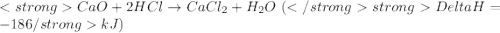 ....(1)
....(1)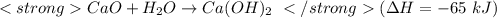
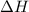 get reversed.
get reversed.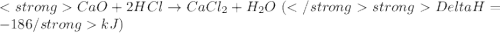 ....(1)
....(1)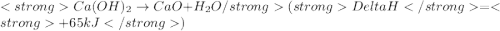 ......(2)
......(2)![Ca(OH)_{2} + 2HCl \rightarrow CaCl_{2} + 2H_{2}O[tex][tex]Delta H = - 186 + 65 = - 121\kJ](/tpl/images/0416/2778/1c69b.png)
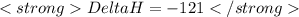 (It is nearly equal to -120.9 kJ)
(It is nearly equal to -120.9 kJ)

