
Chemistry, 13.12.2019 03:31 carlosleblanc26
After distilling your crude methyl benzoate, you set aside 5.12 grams of the purified ester. you then prepare the grignard reagent ( phenylmagnesium bromide ) by reacting 2.3 grams of magnesium with 9.45 ml of bromobenzene. you add the 5.12 grams of methyl benzoate to the freshly prepared grignard reagent to form an addition product. finally, after hydrolyzing the grignard addition product, you obtain 5.3 grams of the final product, triphenyl carbinol. what is the percent yield of triphenyl carbinol ? ( the density of bromobenzene is 1.495 g/ml, triphenyl carbinol = 260.33 g/mol, bromobenzene = 157.01 g/mol, mg = 24.3 g/mol )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
After distilling your crude methyl benzoate, you set aside 5.12 grams of the purified ester. you the...
Questions


Mathematics, 19.11.2019 05:31

Physics, 19.11.2019 05:31

Mathematics, 19.11.2019 05:31

History, 19.11.2019 05:31

Social Studies, 19.11.2019 05:31

Biology, 19.11.2019 05:31

Mathematics, 19.11.2019 05:31

Health, 19.11.2019 05:31



Arts, 19.11.2019 05:31

History, 19.11.2019 05:31



History, 19.11.2019 05:31



Biology, 19.11.2019 05:31

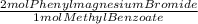 = 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:
= 0.0752 moles of phenylmagnesium bromide. As you have 0.900 moles of phenylmagnesium bromide, limiting reagent is Methyl benzoate and moles of triphenyl carbinol are 0.0376. In grams:

