
Chemistry, 13.12.2019 03:31 bentonknalige
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver nitrate.
calculate the final molarity of chloride anion in the solution. you can assume the volume of the solution doesn’t change when the sodium chloride is dissolved in it.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

You know the right answer?
Suppose 0.245 g of sodium chloride is dissolved in 50. ml of a 18.0 m m aqueous solution of silver n...
Questions

English, 07.05.2021 22:20




Mathematics, 07.05.2021 22:20

Mathematics, 07.05.2021 22:20


English, 07.05.2021 22:20

Mathematics, 07.05.2021 22:20



Mathematics, 07.05.2021 22:20

Mathematics, 07.05.2021 22:20


Mathematics, 07.05.2021 22:20



Mathematics, 07.05.2021 22:20

Computers and Technology, 07.05.2021 22:20

English, 07.05.2021 22:20

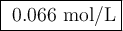
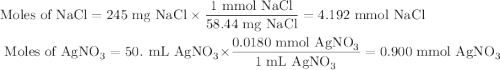
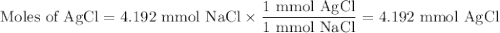
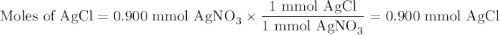
![\text{[Cl$^{-}$] } = \dfrac{\text{3.292 mmol}}{\text{50. mL}} = \textbf{0.066 mol/L}\\\text{The concentration of chloride ion is $\large \boxed{\textbf{0.066 mol/L}}$}](/tpl/images/0416/3996/2071d.png)


