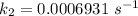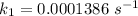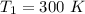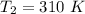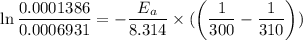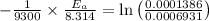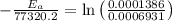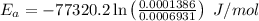Chemistry, 13.12.2019 06:31 supermimi8078
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced to one half of its initial value after 5000s. in contrast, at 310k the conc. is halved after 1000s. using this information to calculate: i) the rate constant for the reaction at 300kii) the time required for the reaction to be reduced to halfiii) the activation energy for the reaction

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Areaction is known to exhibit 1st order kinetics. at 300k the concentration of reactant is reduced t...
Questions

Mathematics, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10


Mathematics, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10


Chemistry, 06.04.2021 22:10


Mathematics, 06.04.2021 22:10

Mathematics, 06.04.2021 22:10



Mathematics, 06.04.2021 22:10

English, 06.04.2021 22:10





Social Studies, 06.04.2021 22:10

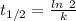
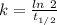
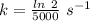
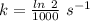
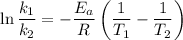

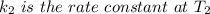
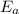 is the activation energy
is the activation energy