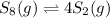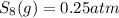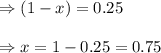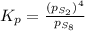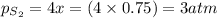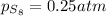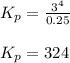1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an in...

Chemistry, 13.12.2019 06:31 cerlos110484
1.
a sample of s8 (8) is placed in an otherwise empty rigid container at 1325 k at an initial pressure of 1.00 atm, where it
decomposes to s2 by the reaction: s8() 4 s2(2). |
at equilibrium, the partial pressure of s, is 0.25 atm. calculate k, for this reaction at 1325 k

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Questions

Mathematics, 22.07.2020 21:01


Mathematics, 22.07.2020 21:01


History, 22.07.2020 21:01

Biology, 22.07.2020 21:01

Mathematics, 22.07.2020 21:01

English, 22.07.2020 21:01


Mathematics, 22.07.2020 21:01

Mathematics, 22.07.2020 21:01

Mathematics, 22.07.2020 21:01




Mathematics, 22.07.2020 21:01



Biology, 22.07.2020 21:01

Mathematics, 22.07.2020 21:01

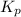 is 324
is 324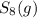 = 1.00 atm
= 1.00 atm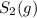 follows:
follows: