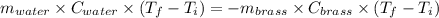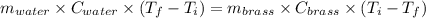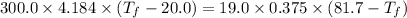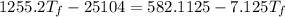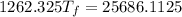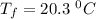Chemistry, 13.12.2019 06:31 BlueLemonWater
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an insulated container containing 300.0g of water at 20.0°c and a constant pressure of 1atm. the initial temperature of the brass is 81.7°c.
1. assuming no heat is absorbed from or by the container, or the surroundings, calculate the equilibrium temperature of the water. be sure your answer has significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1
You know the right answer?
A19.0g sample of brass, which has a specific heat capacity of 0.375·j*g^−1°c−, is dropped into an in...
Questions








History, 29.07.2019 13:30

Mathematics, 29.07.2019 13:30

Health, 29.07.2019 13:30

Arts, 29.07.2019 13:30




English, 29.07.2019 13:30


Mathematics, 29.07.2019 13:30

Mathematics, 29.07.2019 13:30


English, 29.07.2019 13:30