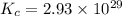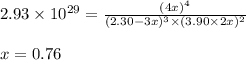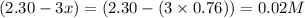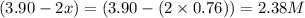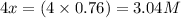Chemistry, 13.12.2019 18:31 zakarycrane9576
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, kc. 3 a ( g ) + 2 b ( g ) − ⇀ ↽ − 4 c ( g ) k c = 2.93 × 10 29 if, at this temperature, 2.30 mol of a and 3.90 mol of b are placed in a 1.00 l container, what are the concentrations of a, b, and c at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions


Mathematics, 09.04.2021 19:20



Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20



Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Biology, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20


Social Studies, 09.04.2021 19:20


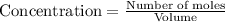
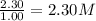
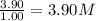
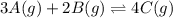
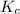 for above equation follows:
for above equation follows:![K_c=\frac{[C]^4}{[A]^3\times [B]^2}](/tpl/images/0417/2973/a92d9.png)