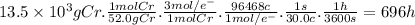Purification of chromium can be achieved by electrorefining chromium from an impure chromium anode onto a pure chromium cathode in an electrolytic cell. how many hours will it take to plate 13.5 kg of chromium onto the cathode if the current passed through the cell is held constant at 30.0 a ? assume the chromium in the electrolytic solution is present as cr 3 +.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
Purification of chromium can be achieved by electrorefining chromium from an impure chromium anode o...
Questions

Spanish, 11.03.2020 02:35

Physics, 11.03.2020 02:35


Computers and Technology, 11.03.2020 02:35

Physics, 11.03.2020 02:35




Social Studies, 11.03.2020 02:35


Biology, 11.03.2020 02:35


Mathematics, 11.03.2020 02:35



Mathematics, 11.03.2020 02:35