
Understanding the high-temperature formation and breakdown of the nitrogen oxides is essential for controlling the pollutants generated by car engines. the second-order reaction for the breakdown of nitric oxide to its elements has rate constants of 0.0796 l/mol-s at 737°c and 0.0815 l/mol-s at 947°c. what is the activation energy of this reaction? give your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Understanding the high-temperature formation and breakdown of the nitrogen oxides is essential for c...
Questions

Mathematics, 17.03.2020 19:04






Geography, 17.03.2020 19:05

Chemistry, 17.03.2020 19:05

Mathematics, 17.03.2020 19:05

Geography, 17.03.2020 19:05


Mathematics, 17.03.2020 19:05



Health, 17.03.2020 19:06



Biology, 17.03.2020 19:06


Biology, 17.03.2020 19:06

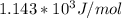
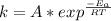
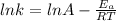
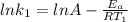 (1)
(1)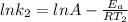 (2)
(2)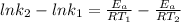 (3)
(3)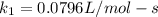 ;
; 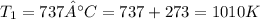
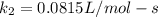 ;
; 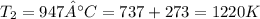
![ln 0.0815 - ln 0.0796 = E_{a}[\frac{1}{8.314*1010} - \frac{1}{8.314*1220}]](/tpl/images/0417/5925/574b6.png)


