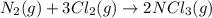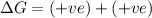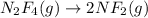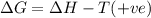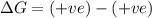Classify each of the following reactions as one of the four possible types:
1. spontan...

Chemistry, 13.12.2019 22:31 jose0765678755
Classify each of the following reactions as one of the four possible types:
1. spontaneous at all temperatures;
2. nonspontaneous at all temperatures;
3. spontaneous at low t; nonspontaneous at high t;
4. spontaneous at high t; nonspontaneous at low t.
(a) n2(g)+3f2(g)→2nf3(g); δh∘=−249kj; δs∘=−278j/k
(b) n2(g)+3cl2(g)→2ncl3(g); δh∘=460kj; δs∘=−275j/k
(c) n2f4(g)→2nf2(g); δh∘=85kj; δs∘=198j/k

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
Questions






Health, 16.11.2020 16:40

Mathematics, 16.11.2020 16:40

English, 16.11.2020 16:40


Mathematics, 16.11.2020 16:40

History, 16.11.2020 16:40


English, 16.11.2020 16:40


Geography, 16.11.2020 16:40

Mathematics, 16.11.2020 16:40

History, 16.11.2020 16:40



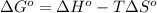
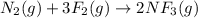
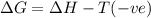
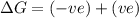
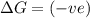 (at low Temperature)
(at low Temperature)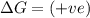 (at high Temperature)
(at high Temperature)